- Product Center
- Anbang News
- Industrial & Commercial Ne..
- Warm Knowledge
- News & Notice
- Trace Heating
- New York heavy snow
- The Northeast American hit by heavy snow,the roof electric heat tracing is very effective
- Contact US
-
TEL:
0086-550-2399006FAX:
0086-550-2399018PHONE:
0086-18355088886EMAIL:Dennis@cnheatingmat.com
After-sales service:Afterservice@anbangcn.com
ADDRESS:
No288.Tianye North Road TianChang City,Anhui,China,239354

Electric vehicle battery technology innovation development is still far off
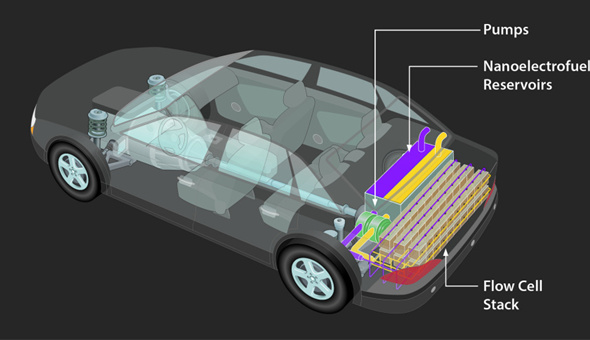
If you were to track the upgrades for your Apple iPhone or Toyota Prius from their introduction to today, you will see a familiar arc in the technology industry: performance multiplies, the product is refined, jobs are created, even entire industries are reworked.
Similarly, Toyota’s Prius hybrid car evolved from a neighborhood oddity (and celebrity eco-accessory) in 2000 to a best-selling vehicle in Japan and California. The engine in today’s model is 20 percent lighter (and offers 20 percent more total horsepower) than the original. Its distance-per-charge is longer. Without the Prius, it can be argued, there would be no Tesla.
Recommend Links:



